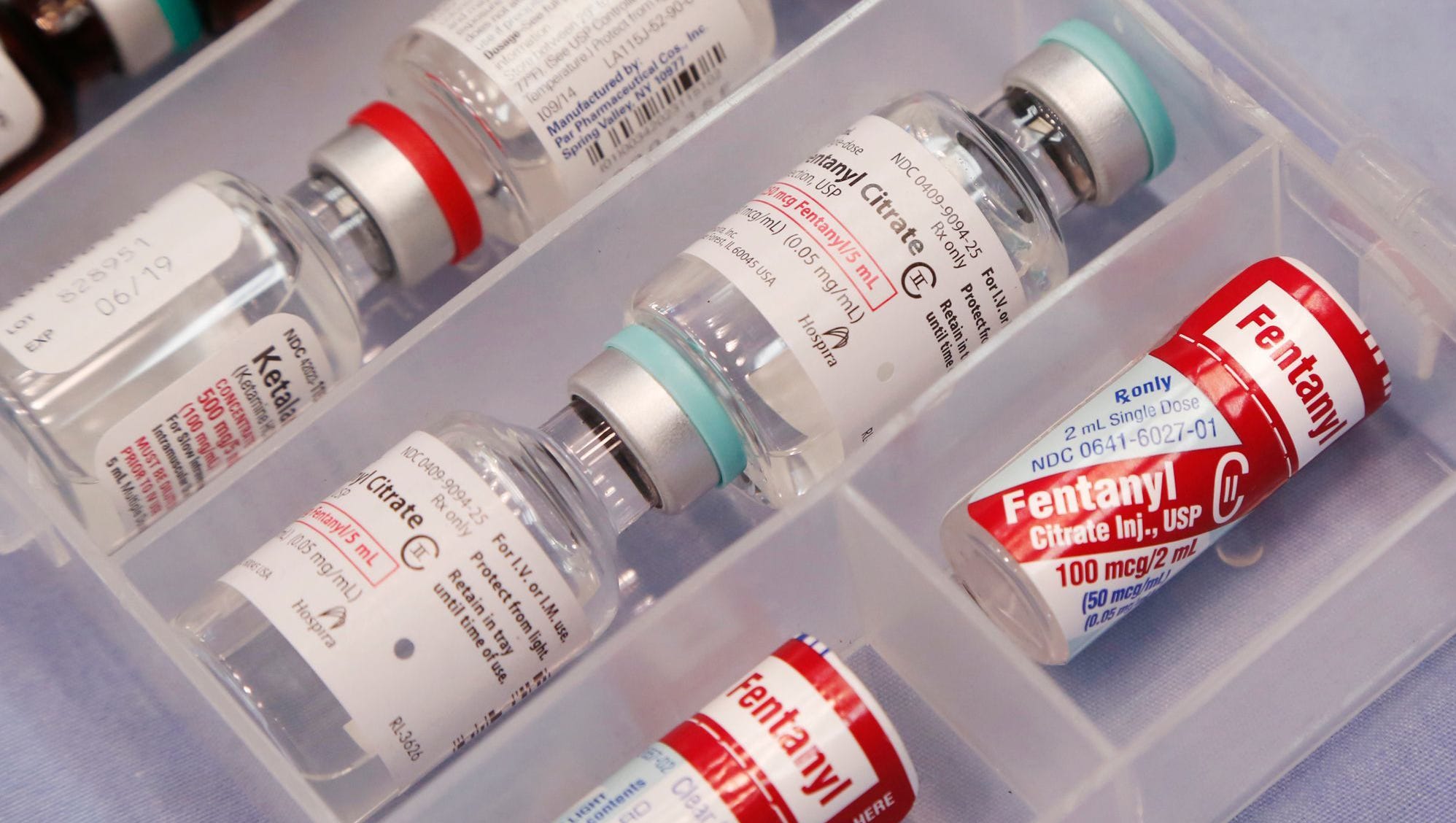
It contains a fentanyl citrate equivalent to a dose of 50 micrograms per milliliter.
Fentanyl citrate, 100 micrograms per 2 ml ampoule, is the active ingredient in each ampoule.
Approximately 500 micrograms of fentanyl citrate are contained in each 10ml ampoule.
Fentanyl citrate, 2500 micrograms per vial, is contained in each vial.
The following are in this medicine:
• Each 2 ml ampoule contains 7.1 mg (or 0.31 mmol) of sodium, making it essentially’sodium-free.’
Each 10 ml ampoule contains 35.4 mg (or 1.54 mmol) of sodium, which is similar to 2% of the WHO’s recommended maximum daily intake for an adult of 2 g sodium.
9 percent of the World Health Organization’s recommended maximum daily consumption of 2 grams of sodium for an adult is found in a 50 ml vial containing 177 mg of sodium.
Opioid analgesic fentanyl is used:
– in modest doses for short surgical procedures to provide analgesic
during life-saving treatment for patients who are dependent on ventilators
using neuroleptics as part of the neuroleptanalgesia approach
myocardial infarction’s painful symptoms can be relieved with the use of opioids
Discuss with patients the need for an end-of-treatment strategy with fentanyl before beginning opioid treatment, in order to minimize the risk of addiction and drug withdrawal syndrome (see section 4.4).
Intravenous bolus or infusion administration.
Subcutaneous injection.
As long as the patient’s airway can be regulated, fentanyl should be administered only in a controlled environment (see section 4.4 Special warnings and precautions for use).
It is suggested that a short intravenous dosage of an anti-cholinergic be administered just prior to anaesthesia induction in order to avoid bradycardia.
Wearing gloves while opening the ampoule is suggested.